Vero Cell Covid Vaccine Efficacy, Sinopharm S Two Covid 19 Shots Effective Study Says Reuters
COVID-19 Vaccine Vero Cell Inactivated detailed edition. 10 11 Peer-reviewed results published in JAMA of Phase III trials in United Arab Emirates and Bahrain showed BBIBP-CorV 781 effective against symptomatic cases and 100 against severe cases 21 cases in vaccinated group vs.

Two Covid 19 Vaccines Approved In China In Less Than 24 Hours 2021 03 02 Bioworld
95 cases in placebo group.

Vero cell covid vaccine efficacy. The woes of tens of thousands of Nepali job seekers whose travel and job plans have been relying on Covid-19 vaccination are not over. When administered by intramuscular inoculation MVA-SARS-2-S expresses and safely delivers the full-length SARS-CoV-2 S protein inducing balanced SARS-CoV-2specific cellular and humoral immunity and protective efficacy in. Efficacy Safety and Immunogenicity of Inactivated SARS-CoV-2 Vaccines Vero Cell to Prevent COVID-19 in Healthy Adult Population In Peru Healthy Adult Population In Peru Covid-Peru The safety and scientific validity of this study is the responsibility of the study sponsor and investigators.
When a person is given the vaccine their immune system identifies the inactivated virus as foreign and makes antibodies against it. The inactivated Vero Cell COVID-19 vaccine is now eligible for global rollout alongside the four other vaccines with World Health Organization Emergency Use Listing. Its easy storage requirements make it highly suitable for low-resource settings.
Vaccine Efficacy 8350 CI 95 6542-9212 Based on symptomatic and RT-PCR positive COVID-19 cases after 14 days and more after the 2nd dose Number of subjects after 14 days and more after the 2nd dose Based on calculation person x year in the follow up period Treatment Arm Number of hospitalized COVID-19 Cases Total Subject Number Person year. Here we provide results from testing the COVID-19 candidate vaccine MVA-SARS-2-S a poxvirus-based vector vaccine that proceeded to clinical evaluation. The SARS-CoV-2 Vaccine VeroCell is an inactivated vaccine against coronavirus disease 2019 COVID-19 which stimulates the bodys immune system without risk of causing disease.
The efficacy rating followed an interim report of ongoing human trials conducted in that country CNBC reported. The vaccine which is developed by Chinas Sinopharm is not recognised by most labour destination countries. The Sinopharm product is an inactivated vaccine called SARS-CoV-2 Vaccine Vero Cell.
Table 1 shows the overall vaccine efficacy of the licensed vaccines after completed vaccination. It is the also first vaccine that will carry a vaccine vial monitor a small sticker on the vaccine vials that change color as the vaccine is exposed to heat letting health workers know whether the vaccine can be safely used. Currently the IHME model uses the following inputs of vaccine efficacy separated by variant.
To determine adverse reactions as a result of pre- and post-exposure rabies vaccination using the conventional intramuscular and reduced dose intradermal regimens and purified Vero cell rabies vaccine. CoronaVac is an inactivated vaccine to be administered intramuscularly as a course of 2 doses each dose of 05ml contains 600SU inactivated SARS-CoV-2 virus as antigen at an interval of 28 days for routine immunization. Vaccinating outbound workers with China-made Vero Cell shots adds to complication officials say.
This is operationalized as a reduction in susceptibility. COVID-19 Vaccine Vero Cell Inactivated also contains an adjuvant a substance that helps strengthen the immune response to the vaccine. Dec 09 2020 By Alex Keown.
The World Health Organization WHO has refuted the claims that a mix and match regimen of Vero Cell and AstraZeneca CoviShield vaccines would yield. So far Vero is the only Chinese vaccine for which the manufacturer has published official data. Once inactivated viruses get presented to the bodys immune system they stimulate the production of antibodies and make the body ready to respond to an infection with live SARS-CoV-2.
Efficacy at preventing symptomatic disease. On December 29 2020 Sinopharm reported 79 efficacy in an interim evaluation. COVID-19 vaccine efficacy from clinical trials COVID-19 vaccines licensed for use in the EUEEA have been shown during clinical trials to be highly effective in providing protection against symptomatic COVID-19 and severe disease.
The COVID-19 vaccine under development by Chinas Sinopharm is showing efficacy of 86 health authorities from the United Arab Emirates reported this morning. A prospective and randomized study of patients exposed to rabies and of subjects in need of pre-exposure rabies vaccination. This is operationalized as a reduction in hospitalization and death.
Estimating vaccine efficacy for COVID-19 projections. It is used for preventing COVID-19 caused by SARS-CoV-2 infection and suitable for people aged 18 years and over for immunization. The World Health Organization WHO has listed Sinopharms Vero Cell COVID-19 vaccine for emergency use allowing it to be rolled out globally.
This vaccine is adjuvanted with. The vaccine was. Its product name is SARS-CoV-2 Vaccine Vero Cell not to be confused with the similar product name of CoronaVac.
Efficacy at preventing infection. A Study to Evaluate The Efficacy Safety and Immunogenicity of Inactivated SARS-CoV-2 Vaccines Vero Cell in Healthy Population Aged 18 Years Old and Above COVID-19 The safety and scientific validity of this study is the responsibility of the study sponsor and investigators. The primary efficacy endpoint is the incidence of symptomatic cases of COVID-19 disease confirmed by RT-PCR two weeks after the second dose of vaccination.
Https Cdn Who Int Media Docs Default Source Immunization Sage 2021 April 1 Sage29apr2021 Sinopharm Pdf Sfvrsn Ddf0d841 5
World Health Organization Who What S The Difference Between Covid 19 Vaccine Efficacy And Effectiveness Vaccine Efficacy Refers To How The Vaccine Performs In Ideal Conditions Controlled Clinical Trials Vaccine
Sinopharm S Two Covid 19 Shots Effective Study Says Reuters

Coronavirus Who Approves Sinovac Covid Vaccine For Emergency Use News Dw 01 06 2021
Sinopharm S Covid 19 Vaccine Shows 86 Efficacy Uae Health Agency Says Biospace
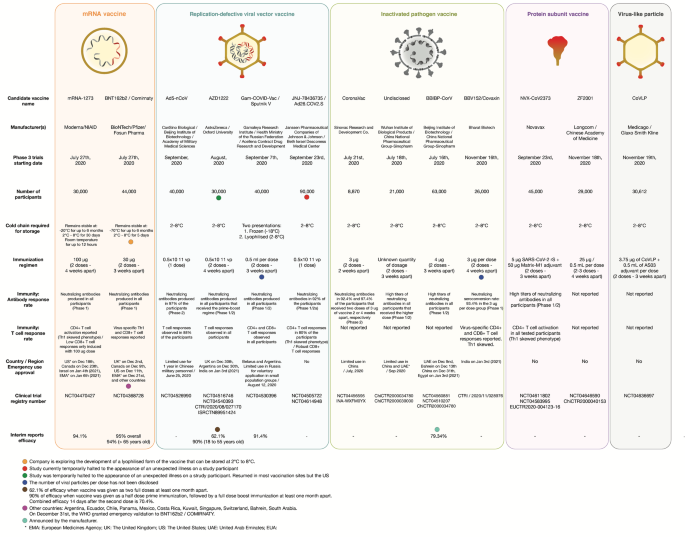
Sars Cov 2 Vaccines Strategies A Comprehensive Review Of Phase 3 Candidates Npj Vaccines

Who Approves China S Sinopharm Covid 19 Vaccine For Emergency Use Has 79 Efficacy Coronavirus Outbreak News

Covid 19 Chinese Official Says Homegrown Vaccines Not Very Powerful Euronews
Egypt Reports No Serious Side Effects On Chinese Covid 19 Vaccine Cgtn

Integrated Control Of Covid 19 In Resource Poor Countries International Journal Of Infectious Diseases

Interim Estimates Of Vaccine Effectiveness Of Bnt162b2 And Mrna 1273 Covid 19 Vaccines In Preventing Sars Cov 2 Infection Among Health Care Personnel First Responders And Other Essential And Frontline Workers Eight U S Locations December

Sinopharm Vero Cell Inactivated Covid 19 Vaccine
Answers For China S First Approved Covid 19 Vaccine Cgtn

China S Covid Vaccine From Sinopharm Is 86 Effective Uae Says

Seychelles Brings Back Curbs Despite Vaccination Success Bbc News

Who Approves A Covid 19 Vaccine From China S Sinopharm For Emergency Use

The Science Behind Chinese Covid 19 Sinopharm Vaccine Inactivated Virus And Mystery Of Falling Immunity Science News

Who Lists Sinopharm S Covid 19 Vaccine For Emergency Use
Coronavirus Covaxin Efficacy Is 81 Works Against Variants The Hindu


Post a Comment
Post a Comment